Link
Conformity with health data privacy regulations is a challenge faced by orthopedic technicians and manufacturing centers (CFAB) alike. Qwadra launches the solution: Link, a highly performant, efficient, data-security compliant platform for your orders and tracking, all in one. This application offers orthopedic technicians a list of manufacturing centers to choose from, to send files and place orders to manufacture their devices with secure data transmission of the patient data and the model to be machined. Another advantage of Link is that it is open, compatible with existing systems. Link works with any STL file, not only Qwadra solution files.
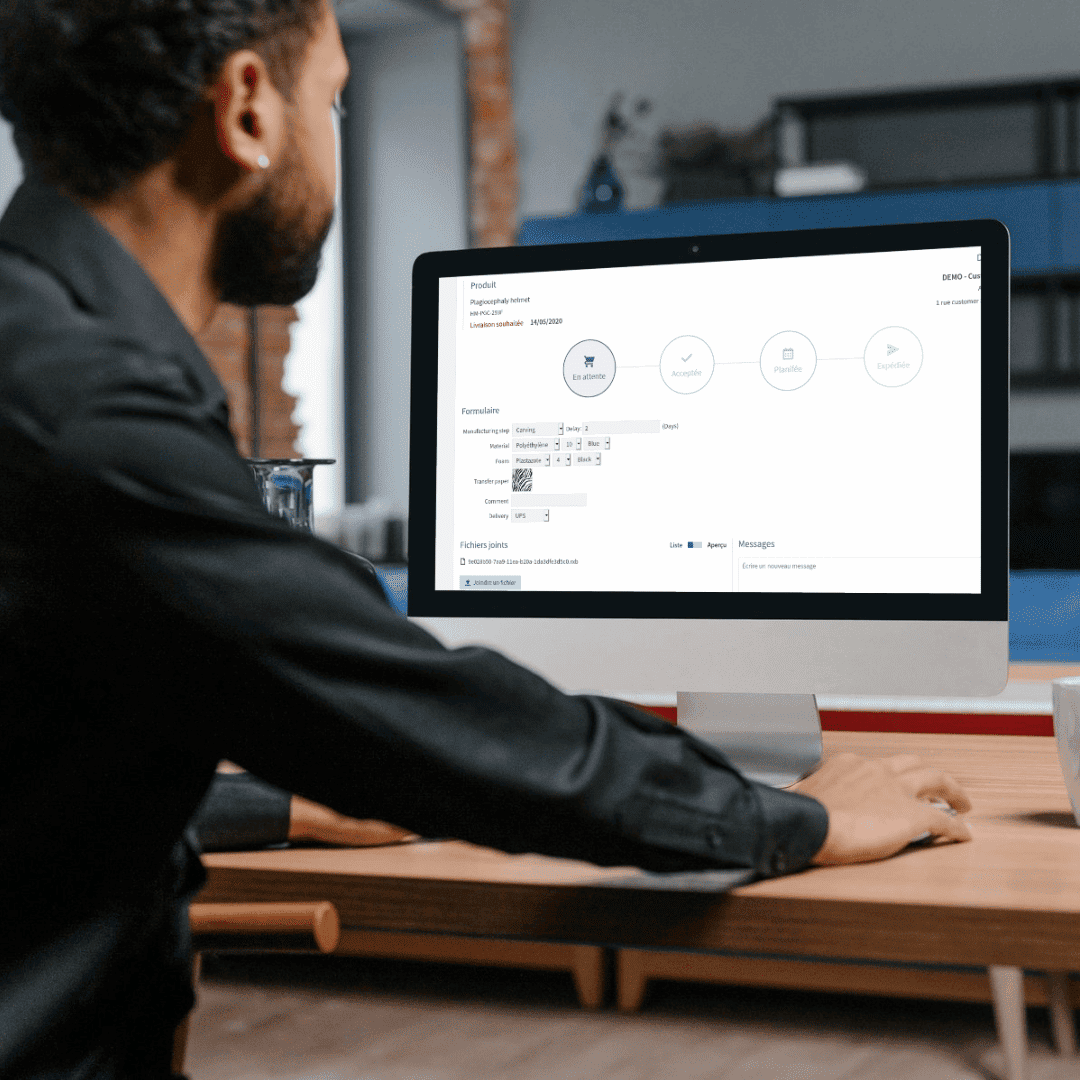
Link: the secure web platform transforming orthopedic manufacturing
In the orthopedic industry, efficiency, security- and compliance are essential when managing orders and manufacturing processes. Orthopedic technicians and manufacturing centers need a reliable solution that ensures secure data transmission and seamless order tracking. Here’s Link, a cutting-edge web platform designed to bridge the gap between orthopedic professionals and manufacturing centers. With a focus on security, interoperability and ease of use, Link streamlines order processing while complying with strict health data privacy regulations. Combined with orthopedic CAD/CAM software, this powerful platform can revolutionize orthopedic device manufacturing.
The link between orthopedic professionals and manufacturers
Link is an innovative web platform that serves as a connection point between orthopedic professionals and manufacturing centers (CFABs). The platform is designed to facilitate seamless order placement and tracking for orthopedic devices, ensuring that technicians and manufacturers can collaborate efficiently.
Orthopedic professionals can browse and select manufacturing centers, choose the necessary materials and fabrication methods, and securely transmit STL files for device production. CFABs, on the other hand, can manage incoming orders, customize product catalogs, and oversee the manufacturing process with real-time updates. Link is built for any organization involved in the design and production of orthopedic devices, ensuring a smooth and effective workflow for all stakeholders.
A secure and compliant platform
One of the primary concerns in the orthopedic sector is ensuring patient data confidentiality while maintaining smooth communication between technicians and manufacturers. Link addresses these challenges with a 100% secure web platform that adheres to GDPR and other health data privacy regulations. This ensures that sensitive patient information, including STL files used for device manufacturing, is transmitted safely and in compliance with industry standards.
By utilizing end-to-end encryption and secure access controls, Link provides orthopedic professionals and CFABs (Centers for the Manufacture of Orthopedic Devices) with peace of mind, knowing their data remains protected. Additionally, the platform supports an unlimited number of orders, allowing businesses to scale operations without compromising security.
From order to delivery: a smoother process for everyone
Beyond security, Link offers several benefits that enhance efficiency and ease of use for both orthopedic professionals and manufacturing centers. As an open platform, Link integrates seamlessly with existing systems and accepts any STL file, eliminating compatibility concerns. The real-time order tracking feature ensures that users can monitor order statuses at every stage, from placement to delivery, providing transparency and efficiency.
Furthermore, central of fabrication can customize their product catalogs and configure manufacturing options, such as materials, colors and techniques, allowing for greater flexibility in the production process. The internal messaging system facilitates seamless communication between technicians and manufacturers, ensuring clear and timely exchanges. With the unlimited order capability, businesses can grow and scale without restrictions, making Link an adaptable and future-proof solution for the orthopedic industry.
The orthopedic industry requires innovative solutions to improve efficiency, maintain compliance and ensure secure data handling. Link by Qwadra offers a comprehensive, secure and interoperable platform that transforms order processing and manufacturing tracking. By enabling seamless collaboration between orthopedic professionals and central fabrication, Link not only enhances workflow efficiency but also ensures high-quality patient care. Whether you’re an orthopedic technician looking for a streamlined ordering solution or a manufacturing center seeking better visibility and management, Link is the future of orthopedic manufacturing connectivity.
Read also :
3Dsizeme
Cube
Fitflow
Canfit
MSoft
Liberty 2
My3Dsizeme
iMed file
Don’t miss the latest updates and news for each of our solutions. You can unsubscribe at any time.
* Please fill out the required fields